Introduction: Detection of measurable residual disease (MRD) prior to allogeneic hematopoietic cell transplant (alloHCT) in patients with acute myeloid leukemia (AML) in remission is associated with increased relapse and inferior survival after transplant. We recently reported that next-generation sequencing (NGS) detection of mutated NPM1 or FLT3-ITD in first complete remission (CR1) blood prior to alloHCT is strongly associated with increased relapse and death (PMID: 36881031). However, the prognostic implications of detecting persistence of other common AML-associated mutations at this treatment landmark remain incompletely defined. Around 20% of patients diagnosed with AML have variants in isocitrate dehydrogenase ( IDH) genes (~7% for IDH1) and clinical outcomes are known to differ due to co-occurrence of other mutations, IDH subtypes, treatment strategies, and patient factors. Studies have examined the relationship between residual IDH1 variants ( IDH1m) in CR and subsequent clinical outcomes, but definitive standardized evidence to inform decision-making for the clinical utility of IDH1m as a target for AML MRD testing was not previously available.
Methods: Adult patients undergoing alloHCT for IDH1 mutated AML in CR1 at CIBMTR sites in the USA between 2013-2019 were eligible for the study if remission blood sample collected within 100 days before alloHCT and outcome data were available. Ultrasensitive error-corrected NGS (NGS-MRD) targeting IDH1, NPM1, and FLT3 genes was performed on DNA from pre-conditioning blood to identify residual variants. Detected IDH1m were validated by digital droplet PCR (ddPCR). The day of transplant was considered as day 0. Median follow-up time was calculated for censored patients. Overall survival (OS) was estimated using Kaplan-Meier (log-rank tests) and Cox proportional hazards models (forward selection); cumulative incidence of relapse was examined using Fine-Gray regression models with non-relapse mortality as a competing risk.
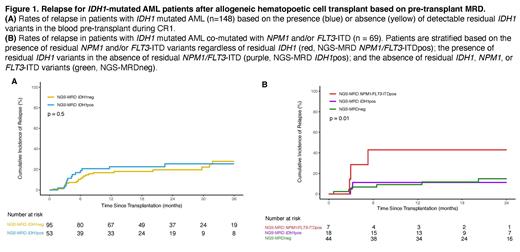
Results: A total of 148 patients were included in the study, with a median follow-up time of 24 months. Relapse was observed in 37 (25%) patients, with the majority occurring within 1 year after alloHCT (n = 28, 76%). Pre-transplant flow cytometry results were reported by the transplant centers for 97% of patients (n = 144), but no statistically significant differences in clinical outcomes were observed between the positive and negative groups (OS: p = 0.3; relapse: p = 0.07). 53 patients (36%) tested positive for IDH1m persistence in CR1, with a 100% validation rate by ddPCR for variants with an orthogonal assay available. Logistic regression indicated that IDH1m persistence in remission was more likely to occur in patients older than 40 years (OR: 1.02 with every 1-year increase after 40, p = 0.04). Clinical outcomes showed no statistically significant difference between the pre-alloHCT IDH1m positive and negative groups (Figure 1A), and this remained true after further subgroup analysis by age. The variant allele fraction (VAF) of the residual IDH1m ranged from 0.09% to 48.5% (median: 1.5%), and no differences in clinical outcomes were observed when stratifying by high (≥2.5%) or low VAF (<2.5%). Among patients with co-mutated NPM1 and/or FLT3-ITD at baseline (n=69), those with residual mutated NPM1 and/or FLT3-ITD in pre-transplant remission had significantly higher rates of relapse compared to those with residual IDH1m only or NGS-negative groups (p = 0.01, Figure 1B). The cohort excluding patients with co-mutated NPM1 and/or FLT3-ITD did not show any significant clinical outcome differences based on IDH1m persistence. Conditioning intensity did not show an association with clinical outcomes, and multivariable analysis for overall survival and relapse did not identify residual IDH1m as an important marker.
Conclusion: Detection of persistent IDH1 variants in the blood of adult patients with AML in CR1 prior to first alloHCT is common, but not associated with increased relapse or death after transplant compared with those testing negative. For those patients with IDH1 mutated AML co-mutated with either NPM1 and/or FLT3-ITD, detection of persistent NPM1 and/or FLT3-ITD was associated with higher rates of relapse. These findings, from the largest study to date, do not support the detection of isolated IDH1 variants in CR1 blood prior to alloHCT as evidence of AML MRD or increased post-transplant relapse risk.
Disclosures
Andrew:Astra Zeneca: Current Employment. Auletta:National Marrow Donor Program: Current Employment; Takeda: Membership on an entity's Board of Directors or advisory committees; AscellaHealth: Membership on an entity's Board of Directors or advisory committees. El Chaer:BioSight: Research Funding; PharmaEssentia: Research Funding; MEI Pharma: Research Funding; Sanofi: Research Funding; Sumitomo Pharma Oncology: Consultancy, Research Funding; Bristol Myers Squib: Research Funding; Amgen: Consultancy, Research Funding; Fibrogen: Research Funding; Celgene: Research Funding; Association of Community Cancer Centers: Consultancy; DAVA Oncology: Other: Travel grant; Novartis: Research Funding; Arog Pharmaceuticals: Research Funding. Chen:Rigel: Consultancy; Abbvie: Consultancy. Corner:Bio-Rad Laboratories: Current Employment, Current holder of stock options in a privately-held company. Jimenez Jimenez:Abbvie: Research Funding. de Lima:Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Scribb: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Data Safety Monitoring Board; AbbVie: Other: Data Safety Monitoring Board; Miltenyi Biotec: Research Funding. Kebriaei:Pfizer: Consultancy, Honoraria; Jazz: Consultancy, Honoraria. Hourigan:Foundation of the NIH AML MRD Biomarkers Consortium: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal